MONTREAL, QC – December 16, 2024 – MY01, an innovative medical technology company dedicated to revolutionizing the diagnosis of compartment syndrome, proudly announces the shipment of its 2,000th device. This significant milestone highlights the company’s dedication to improving patient outcomes by providing real-time, continuous pressure monitoring that aids healthcare professionals in making timely, objective decisions.
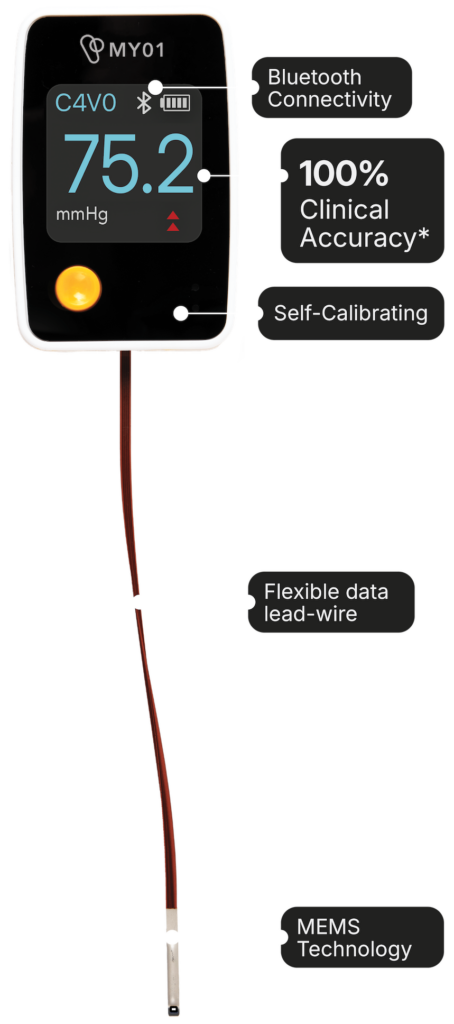
The MY01 device, powered by advanced Microelectromechanical Systems (MEMS) technology, offers continuous intracompartmental pressure monitoring, transforming the traditionally subjective and often delayed diagnosis of compartment syndrome. This technology is vital in trauma care as it provides reliable and accurate pressure tracking, enabling faster intervention and reducing complications.*
Charles Allan, CEO of MY01, commented, “Reaching the 2,000th device mark is a momentous achievement for MY01. It demonstrates the medical community’s trust in our solution to make a tangible difference in patient care. We’re thrilled to see our devices becoming an indispensable tool in hospitals across North America and beyond.”
As MY01 celebrates its 2,000th device, its mission remains steadfast: to provide healthcare professionals with actionable, real-time data that can lead to better clinical decisions and improved patient outcomes. Each device shipped represents a step forward in making compartment syndrome diagnosis faster, more accurate, and more reliable.
About MY01, Inc
Founded in 2019 and based in Montreal, MY01 envisions a world where every disease is quantifiable, enabling precise, personalized care for all patients. Specializing in managing compartment syndrome, our tools provide proactive monitoring and actionable insights through a connected care team that benefits patients, providers, and payers. MY01 is commercial in the USA, Europe, and Canada, setting new standards in healthcare.
*Data presented at the Orthopedic Trauma Association Annual Meeting, Seattle, 2023. (ClinicalTrials.gov ID NCT04012723)